NAC Attack: A Multicenter, Placebo-Controlled Clinical Trial To Test Oral N-Acetylcysteine in Patients
NAC is a strong antioxidant that reduces oxidative damage. NAC Attack is a randomized, placebo-controlled multicenter trial that will test whether NAC can slow progression of RP. The results are critical to the RP community because if NAC proves beneficial it will mean lifelong treatment with NAC for patients with RP. We understand the anxiety and concerns that RP causes, but it is best to avoid self-medication with supplements.
Frequently Asked Questions
-
Retinitis pigmentosa (RP) is an inherited retinal disease resulting from one or multiple genetic mutations. The mutations cause death of photoreceptor cells, resulting in the first symptom of loss of night vision. Then gradually, patients start to notice constricted visual fields followed by loss of visual acuity over years or decades until legal blindness.
RP is one of the most common causes of blindness and severe low-vision in people aged 20 to 60 years. The estimated population prevalence is 1 in 3500-4000 in the US and Europe. Currently, there is no therapeutic treatment for RP.
-
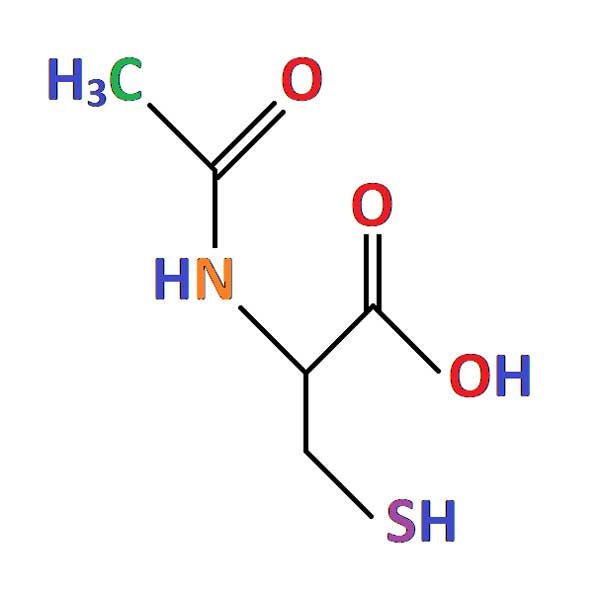
N-acetylcysteine (NAC) is a plant antioxidant that can be naturally found in onions. It is an FDA approved medication currently used to treat acetaminophen (paracetamol) overdose and as a mucolytic for cystic fibrosis and other pulmonary diseases.
-
NAC Attack is a Phase III multicenter, randomized, placebo-controlled study to evaluate the efficacy and safety of oral NAC in patients with RP.
Compelling studies have proven that excessive oxidative stress plays a key role in photoreceptor cell death in RP. The effect of NAC treatment on reducing photoreceptor loss has been shown in animal studies.
The safety profile of oral NAC treatment is well known for short term use and was recently demonstrated in 30 patients with RP who received oral NAC for 6 months. A multicenter, randomized, placebo-controlled clinical trial in a larger population from different places of the world and over a longer time is necessary to determine the long-term safety of NAC and determine whether oral NAC can slow photoreceptor loss and thus delay visual function loss in patients with RP.
-
A randomized, placebo-controlled trial means that study patients are randomly assigned to receive the experimental intervention treatment or the placebo that resembles the intervention treatment but does not contain the active intervention component.
Because the assignment is random, patients receiving the intervention and patients receiving the placebo are expected to be very similar. Thus, any difference between the two patient groups with respect to disease related outcomes is most likely due to the treatment the patient received (i.e. intervention or placebo control). Such a study design (“randomized, placebo-controlled” clinical trial) is known to be the “gold standard” for determining whether the intervention indeed works for the disease.
-
No, we do not yet know whether NAC is truly helpful and not harmful, so we do not recommend taking it outside of a closely monitored situation like a study. In addition, the NAC available in drug stores or online is supplement grade NAC which is not regulated, meaning that the producers do not have to measure exactly what is in it. When investigators have tested commercially available supplement grade NAC, they have found that it often does not contain the amount of NAC advertised and sometimes not much at all. In June 2020, the FDA issued warning letters to industry banning NAC in supplements.
If you would like to be a part of the trial and are currently taking NAC, you must stop it and be off it for at least 4 months to be eligible to enter the trial.
-
The NAC Attack study along with the trial sponsor (the National Eye Institute, one institute of the National Institutes of Health of the U.S. Department of Health and Human Services) has identified approximately 30 clinics that specialize in inherited retinal degenerations to participate in the trial as clinical sites. (See Clinical Sites: Locations and Contact Information.) Enrollment has commenced in 23 sites as of spring 2024 including 20 US and 3 international sites in Canada, Germany and the Netherlands. More sites are expected to start enrollment very soon.
You may contact the site that is convenient for you now and inquire about participating in the trial. If you are potentially eligible, you may receive a pre-screening phone call from the site’s study coordinator and if deemed potentially eligible based on the study enrollment criteria, you will be scheduled to attend an in-clinic screening visit. With your consent, this visit will collect certain information about your general health and your eye conditions to help determine whether you fit all the enrollment criteria. If you have not been seen at this clinic before, you may be guided by the study coordinator to bring in medical records related to your diagnosis of RP from elsewhere when you attend the in-clinic screening visit.
NAC Attack Video FAQ
English | Closed captioning is available by clicking the "CC" icon in the bottom right corner. Select the language you want from the list of available languages.
Nederlands | Tik op Ondertiteling "CC." Selecteer de taal waarin je de ondertiteling wilt zien.
Français | Appuyez sur Sous-titres "CC ". Sélectionnez la langue dans laquelle vous souhaitez afficher les sous-titres.
Deutsch | Tippe auf das Untertitelsymbol "CC". Wähle die Sprache aus, in der die Untertitel angezeigt werden sollen.
Español | Toca Subtítulos "CC". Elige el idioma en el que quieras ver los subtítulos.
NAC Attack | Important Things To Remember
NAC Attack | Caring for Your Medication
NAC Attack | Using the Medication
NAC Attack | What To Expect at Your Health-care Visits
NAC Attack | What To Do if You Have a Question
NAC Attack Sponsor
Supporting Organizations
- Zambon SpA, Milan, Italy
- Usher 2020
- Fighting Blindness Canada
- Retina UK
NAC Attack Leadership
Study Chairman's Office
- Study Chair: Peter Campochairo, M.D.
- Vice Chair: Mandeep Singh, M.B.B.S., M.D., Ph.D.
- Vice Chair: Rupert Strauss, M.D.
- Gulnar Hafiz
- Dagmar Wehling
Coordinating Center
- Director and Senior Biostatistician: Xiangrong Kong, Ph.D.
- Informatics Director: Kurt Dreger
- Project Coordinators: Yanzhao (Izzie) Wang and Chrisanthe Papapavlou
- Finance: Simone Butler and Lee Rorke
- Research Fellow: Folahan Ibukun
- Research Assistant: Guoshu Kong
NAC Attack Data Safety Monitoring Committee (DSMC)
Chair: Steven Piantadosi, M.D., Ph.D.
Biostatistics and Oncology, Brigham and Women's Hospital, Harvard University
Elise Heon, M.D.
Ophthalmology and Vision Sciences, University of Toronto
Judith Hochman, M.D.
Cardiology and Clinical Trials, New York University
David C. Musch Ph.D., M.P.H.,
Epidemiology and Ophthalmology, University of Michigan
Stephen H. Tsang, M.D., Ph.D.
Ophthalmology, and Pathology and Cell Biology, Columbia University
David N. Zacks, M.D., Ph.D.
Ophthalmology and Visual Sciences, University of Michigan
Alison Bateman-House, Ph.D.
Population Health, New York University