Laboratory of Genetic Neurobiology
Director: Russell L. Margolis, M.D.
The goal of investigations directed by Dr. Margolis is to improve the understanding and treatment of neuropsychiatric disorders, leveraging insights from monogenic neuropsychiatric disorders such as Huntington’s disease, to investigate complex disorders, particularly schizophrenia. Conceptually, the work in the lab is based on the perspective that a bottom-up perspective, in which genetic and environmental etiological factors lead to abnormalities in cell and circuit function, which in turn lead to subsequent clinical syndromes, provides the most robust blueprint for advancing research.
Projects
-
- Andor Bodnar, Ph.D.
- Steven Doll, Ph.D.
- Hanna Jaaro-Peled, Ph.D.
- Claire McCalley, B.A.
- Ethan Mondell, B.A.
- Rachel Scheinberg, B.A.
- Zhan Za, B.A.
-
Johns Hopkins
- Krista Baker, L.C.P.C.
- Wanzhen Duan, M.D., Ph.D.
- Jun Hua, Ph.D.
- Jing Jin, Ph.D.
- Pan Li, Ph.D.
- Chan-Hyun Na, Ph.D.
- Frederick Nucifora, D.O, Ph.D. M.H.S.
- Leslie Nucifora, Ph.D.
- Adrian Paez, M.S.
- Tilak Ratnanther, Ph.D.
- Christopher A. Ross, M.D., Ph.D.
- Juan Troncoso, M.D.
- Max Wolcott, M.S.W.
- Peter van Zijl, Ph.D.
Elsewhere
- David Anderson, MB BcH, Ph.D. (Univ of Glasgow)
- Melanie Bennett, Ph.D. (Univ of Maryland)
- Amanda Krause, MB BcH, Ph.D. (Univ of Witwatersand)
- Vanessa Wheeler, Ph.D. (Harvard Univ/MGH)
- Xiping Zhan, Ph.D. (Howard University
Project 1: The pathogenesis of HDL2

HDL2, discovered and genetically defined by our group, is an adult onset autosomal dominant neurodegenerative disorder, characterized by abnormalities of movement, cognition and emotion with relentless progression until death. It appears to occur exclusively among individuals of African descent. The clinical, neuroimaging, and neuropathological profile of HDL2 is remarkably similar to HD. HDL2 is caused by a CTG/CAG expansion on chromosome 16q24. In the CTG orientation, the HDL2 repeat falls in exon 2A of the gene junctophilin-3 (JPH3; mouse = JP3). An antisense transcript contains an open reading frame that encodes a short peptide that includes polyglutamine.

Our long-term goal is to determine the unique and shared pathogenic mechanisms shared by HD and HDL2. We propose that shared pathways will provide a focus for detecting and validating therapeutic targets relevant to both diseases, while pathways unique to HDL2 will lead to further understanding of the pathogenesis of repeat expansion diseases, with implications for other neurodegenerative diseases. Our investigations are facilitated by access to human HD and HDL2 postmortem brain tissue, iPSCs, and several mouse models, as well as a broad range of collaborators.

Our guiding hypothesis is that HDL2 arises from a combination of loss of JPH3 function, RNA toxicity, and toxicity from proteins with expanded amino acid tracts. Current projects include the impact of repeat length on transcript expression, the differential extent and mechanisms of somatic expansion in HD and HDL2, HD vs HDL2 proteomics, characterization of a new HDL2 knock-in mouse model, the behavioral and electrophysiological impact of loss of JPH3 expression, the potential interaction of JPH3 and HTT, and potential HDL2 therapeutic mechanisms.
Selected References relevant to HDL2:
- Holmes SE, O’Hearn E, Callahan C, Hwang HS, Rosenblatt A, Ingersoll-Ashworth RG, Fleisher A, Stevanin G, Brice A, Potter NT, Ross CA, Margolis RL. A CTG trinucleotide repeat expansion in Junctophilin 3 is associated with Huntington's Disease-Like 2 (HDL2). Nature Genetics, 29 (2001): 377-378.
- Rudnicki DD, Holmes SE, Lin M, Thorton CA, Ross CA, Margolis RL. Huntington’s disease-like 2 is associated with CUG repeat containing RNA foci. Annals of Neurology, 61 (2007):272-82.
- Rudnicki DD, Pletnikova O, Vansattel JP, Ross CA, Margolis RL. A comparison of Huntington’s disease and Huntington’s disease-like 2 neuropathology. Journal of Neuropathology and Experimental Neurology, 67(2008):366-74.
- Seixas AI, Holmes SE, Takeshima H, Pavlovich A, Sachs N, Pruitt JL, Silveira I, Ross CA, Margolis RL (co-corresponding author), Rudnicki DD. Loss of junctophilin-3 contributes to Huntington’s Disease-like 2 pathogenesis, Annals of Neurology, 71(2012):245-257.
- Rudnicki DD, Margolis RL. Pathogenic insights from Huntington's disease-like 2 and other Huntington's disease genocopies. Curr Op Neurology, 29(2016):743-748.
- Khaled HG, Feng H, Hu X, Sun X, Zheng W, Li PP, Rudnicki DD, Ye W, Chen Y-c, Southall N, Marugan J, Ross CA, Ferrer M, Henderson MJ, Margolis RL. High-throughput screening to identify small molecules that suppress huntingtin promoter activity or activate huntingtin-antisense promoter activity. Sci Rep. 11(2021):6157.
- Li PP, Margolis RL. Use of single guided Cas9 nickase to facilitate precise and efficient genome editing in human iPSCs. Sci Rep. 11(2021):9865
Project 2: Schizophrenia neurogenetics
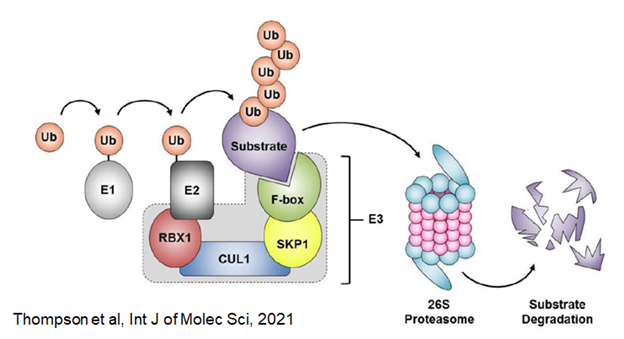
The Lab has a track record of detecting and exploring rare mutations with potential major impact on schizophrenia. Our current focus is on rare loss of function mutations in Cul1 (cullin 1), which the SCHEMA consortium has reported increases the risk of developing schizophrenia by ~30x. The Cul1 protein product encodes an important scaffolding protein in the ubiquitin-ligase complex that is central to protein normal protein degradation.
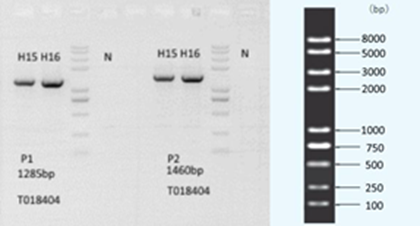
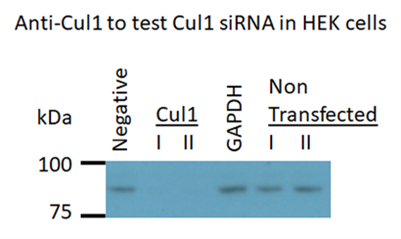
We hypothesize that loss of Cul1 expression will disrupt protein homeostasis, and that the mechanisms involved may provide mechanistic insight into the recent finding by the Nucifora lab of accumulations of insoluble protein in brains and cells of a subset of individuals with schizophrenia. We are currently developing cell knockdown models and a conditional mouse knockout model to test this hypothesis.
Selected references relevant to schizophrenia neurogenetics:
- Sachs NA, Sawa A, Holmes SE, Ross CA, DeLisi LE, Margolis RL. A frameshift mutation in Disrupted in Schizophrenia 1 segregates with schizophrenia and schizoaffective disorder in an American family. Molecular Psychiatry 10(2005) 758-64.
- Chiang C-H, Su Y, Wen Z, Yoritomo N, Ross CA, Margolis RL (co-corresponding author), Song H, Ming G-l. Integration-free induced pluripotent stem cells derived from schizophrenia patients with a DISC1 mutation. Molecular Psychiatry 16 (2011):358-60.
- Yu L, Arbez N, Nucifora LG, Sells G, DeLisi L, Ross CA, Margolis RL, Nucifora FC. A mutation in NPAS3 segregates with mental Illness. Molecular Psychiatry 19(2014):7-8.
- Nucifora LG, MacDonald ML, Lee BJ, Peters ME, Norris AL, Orsburn BC, Yang K, Gleason K, Margolis RL, Pevsner J, Tamminga CA, Sweet RA, Ross CA, Sawa A, Nucifora FC Jr.Am J Psychiatry. Increased Protein Insolubility in Brains From a Subset of Patients With Schizophrenia. American J Psychiatry. 176(2019):730-743.
Project 3: Neuroimaging and schizophrenia
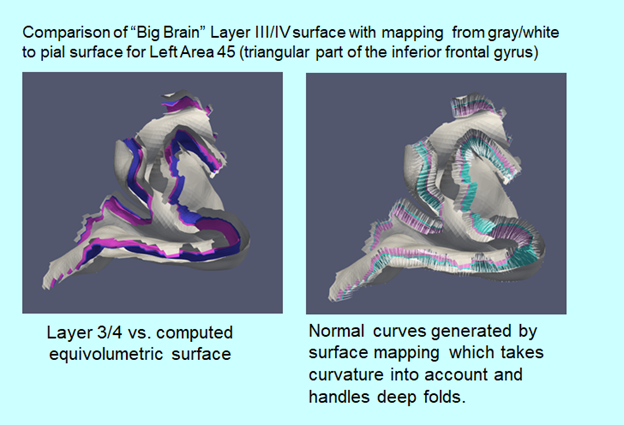
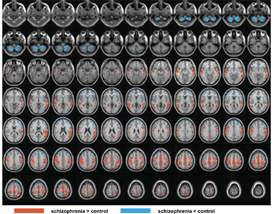
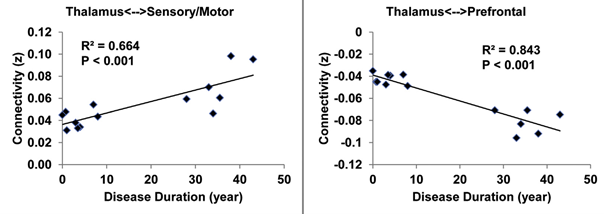
In conjunction with Dr. Jun Hua and colleagues at the Kirby Center for Functional Brain Imaging of the Kennedy Krieger Institute, the lab is using the Kirby Center‘s powerful 7 Tesla scanner to examine anatomical, vascular, and functional abnormalities in schizophrenia. We are focusing on recent-onset, treatment resistant, and ultra-treatment resistant schizophrenia. Dr. Hua has developed novel methods for assessing arteriolar blood volume (iVASO) and brain lymphatic flow that the lab is applying to schizophrenia. Under the leadership of collaborator Dr. Tilak Ratnanather of the Department of Biomedical Engineering and the Center for Imaging Science, diffeomorphometric analysis of cortical thickness is under development to accurately quantify cortical thickness for use in layer specific fMRI, with an emphasis on thalamo-cortical pathways.
Selected References:
- Reading SAJ, Oishi K, Redgrave GW, McEntee J, Shanahan M, Yoritomo N, Younes L, Mori S, Miller MI, van Zijl P, Margolis RL (corresponding author), Ross CA. Diffuse abnormality of low to moderately organized white matter in schizophrenia. Brain Connectivity, 1(2011):511-9.
- Brandt AS, Unschuld PG, Pradhan S, Lim IA, Churchill G, Harris AD, Hua J, Barker PB, Ross CA, van Zijl PC, Edden RA, Margolis RL. Age-related changes in anterior cingulate cortex glutamate in schizophrenia: A (1)H MRS Study at 7Tesla. Schizophr Res. 172(2016):101-5.
- Hua J, Brandt AS, Lee S, Blair NI, Wu Y, Lui S, Patel J, Faria AV, Lim IA, Unschuld PG, Pekar JJ, van Zijl PC, Ross CA, Margolis RL. Abnormal Grey Matter Arteriolar Cerebral Blood Volume in Schizophrenia Measured With 3D Inflow-Based Vascular-Space-Occupancy MRI at 7T. Schizophr Bull. 43(2017):620-632.
- Hua J, Blair NIS, Paez A, Choe A, Barber AD, Brandt A, Lim IAL, Xu F, Kamath V, Pekar JJ, van Zijl PCM, Ross CA, Margolis RL. Altered functional connectivity between sub-regions in the thalamus and cortex in schizophrenia patients measured by resting state BOLD fMRI at 7T. Schizophr Res. 206(2019):370-377.
Project 4: Schizophrenia and Electroencephalography (EEG)

EEG has the advance of detecting both base line neural activity and rapid brain responses to various stimuli. We are launching a new project, using EEG to held identify subtypes of schizophrenia based on treatment response. Stimuli include a variety of auditory stimuli, including oddball tasks and spatial tasks. In addition, we will use EEG recordings, in conjunction with cognitive testing, to generate the Biotypes developed by the B-SNIP consortium, and apply the Biotypes to schizophrenia subtypes defined by treatment response.
Project 5: Schizophrenia diagnosis and treatment

Dr. Margolis, with Division of Neurobiology colleague and long-term collaborator Dr. Frederick Nucifora, are the senior psychiatrists for the four clinics providing care for people with schizophrenia, schizoaffective disorder, and related conditions at Johns Hopkins Bayview. On-going research integrates the study of these patients, and data mined from the Johns Hopkins Medical System, with other activities of the Margolis Lab, the Nucifora Lab, and the Schizoaffective Disorder Precision Medicine Center of Excellence Directed by Dr. Margolis and co-Directed by Dr. Nucifora. Projects include determining the frequency and context of misdiagnosis of schizophrenia, the value of outpatient consultation services, the relationship of cognitive and clinical features of patients with treatment response and clozapine use. We are also part of a NIMH consortium, termed the Connection Learning Health System that systematically collects data from every clinic specializing in the care of individuals with first episode psychosis in Maryland and Pennsylvania. We are using this data to examine clozapine use in this population, and other data from the consortium is available to us for new projects.
Selected References:
- Andrews CE, Baker K, Howell CJ, Cuerdo A, Roberts JA, Chaudhary A, Lechich S, Nucifora LG, Vaidya D, Mojtabai R, Margolis RL, Sawa A, Nucifora FC Jr. Risk of Hospitalization Due to Medication Nonadherence Identified Through EMRs of Patients With Psychosis. Psychiatr Serv. 68(2017):847-850.
- Nucifora FC Jr, Baker KK, Stricklin A, Cuerdo A, Parke KR, DuBois S, Nucifora LG, Margolis RL, Sawa A, Harvey PD. Better functional capacity and cognitive performance in clozapine responders compared to non-responders: A cross-sectional study. Schizophr Res. S0920-9964(2020):30558-2.
- Coulter C, Baker K, Margolis RL. Specialized consultation for suspected recent-onset schizophrenia: diagnostic clarity and the distorting impact of anxiety and reported auditory hallucinations. J Psychiatr Prac, 25(2019):76-81.
