Researchers at Johns Hopkins All Children's Discover a Novel Pathway that is Critical for Muscle Regeneration Following Injury

Andreas Patsalos, M.S., Ph.D., (left), and Laszlo Nagy, M.D., Ph.D.
Laszlo Nagy, M.D., Ph.D. a professor of medicine in the Division of Endocrinology, Diabetes and Metabolism in the Departments of Medicine and Biological Chemistry in the Johns Hopkins University School of Medicine, focuses much of his research on identifying and understanding how the “identity” of cells develops. He is interested in discovering how their differentiation and response to different environmental cues contribute to human diseases. That interest has led him and his colleagues to investigate the relationship between various cell functions and diseases.
“I have always been fascinated by the processes how cells ‘decide’ what to do,” Nagy says. “All cells are essentially the same genetically, whether they are brain cells or liver cells, but they use information differently. Cells called ‘macrophages’ are a good example. They are typically known as a type of white blood cell that helps eliminate pathogens by engulfing them and initiating a response from the immune system.”
As associate director of the Johns Hopkins Center for Metabolic Origins of Disease, one of Nagy’s core research efforts is aimed at getting a better understanding of how cells use certain “pieces” of genetic information and not others and what causes that differentiation process at times to result in diseases causing chronic inflammation and tissue degeneration.
For Nagy and his colleagues, it is important to better understand how macrophages play a key role in protecting against pathological conditions. They are also interested in how an imbalance of macrophages can compromise the immune system and their function during an inflammatory response.
Accordingly, his research interests include searching for better ways to treat muscle tissue damage through enhancing a process called the “Regeneration-Promoting Program” (RPP).
“Regenerative inflammation is a relatively new term,” Nagy explains. “RPP is distinguished by a response to injury in which the damage-caused inflammation helps tissue to start healing. This type of inflammation is useful and essential, and we are interested in gaining a better understanding of how macrophages help repair injured tissues in the body.”
Nagy and the researchers in his lab want to better understand how cells change their nature and transition to doing different “jobs” in the body — in this case; the job at hand is responding to injury and, at the same time, mounting an inflammatory response that will help muscles to heal and regenerate.
“The immune system is emerging as a critical regulator of many processes,” Nagy says. “That includes skeletal muscle regeneration. Inflammation is not generally considered to be something good, but inflammation has tremendous healing potential — it’s a double-edged sword.”
Studying GDF-15
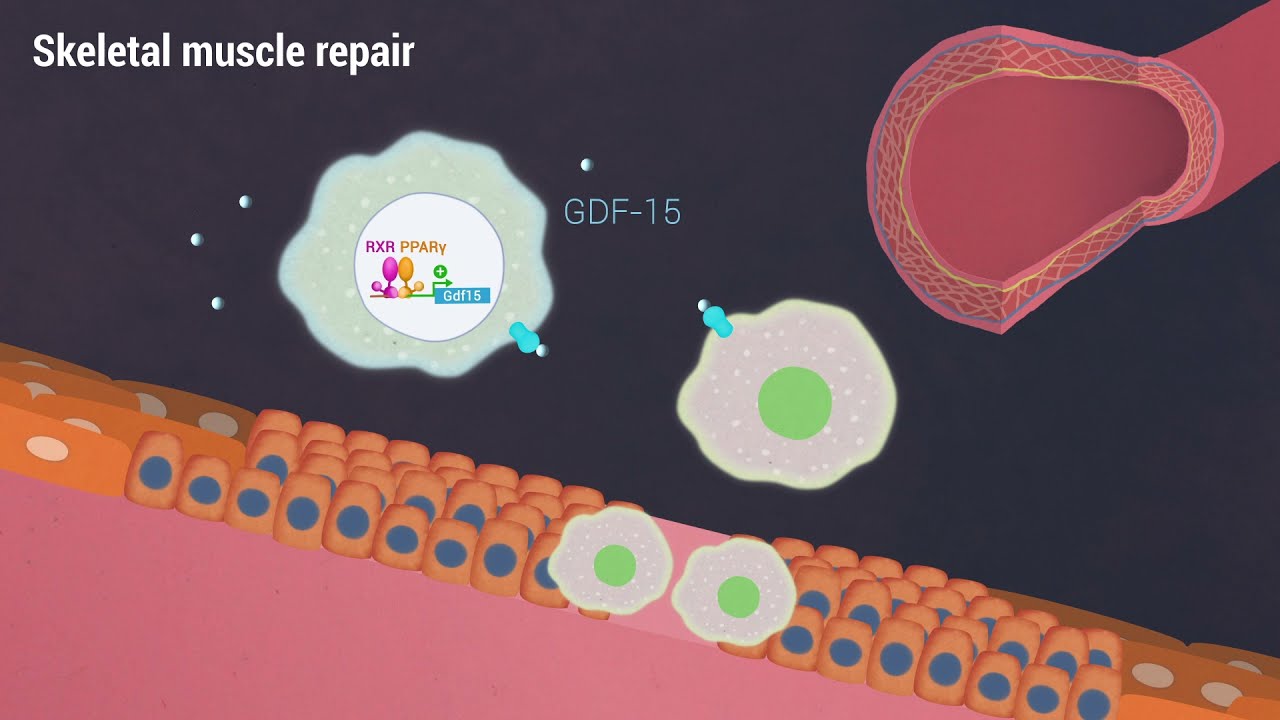
Recent research has focused on how a damaged muscle can begin healing when macrophages rush in and secrete growth factors, including growth/differentiation factor-15 (GDF-15). It is a process that begins when the immune system senses inflammation and damage and speeds macrophages to the injury site, and healing soon begins.
Their study, titled “A growth factor-expressing macrophage subpopulation orchestrates regeneration inflammation via GDF-15,” was published recently in The Journal of Experimental Medicine, a top-tier journal established 125 years ago by William H. Welch, M.D., a founding professor of The Johns Hopkins Hospital, and now owned by Rockefeller University Press. They investigated how specific macrophage subtypes secrete GDF-15 to initiate the process of the RPP and how the knowledge of this process might help develop better treatments for muscle damage.
According to Nagy, growth factors function as “signaling molecules” between cells. GDF-15 is a novel cytokine secreted by unique macrophage subtypes. The family of GDF proteins is important during embryonic development, particularly in the skeletal, nervous and muscular systems.
To carry out their study, the researchers used mouse models of acute damaged muscle tissue and observed how inflammation was accompanied by a “rapid and robust” infiltration of growth factor-expressing macrophages. Healing began immediately.
“The results of our study indicate that macrophages are the predominant — if not the only — source of GDF-15 and other growth factors within the injured tissue,” Nagy summarizes. “It will be important to uncover the receptors and pathways that enable and mediate the activity of these factors after they are secreted.”
In their paper, the researchers noted there were “wide associations of GDF-15 with a variety of biological processes, including pregnancy, metabolism, and inflammation” and that it is highly likely that GDF-15 plays additional systemic roles in the body beyond those described in their study.
“GDF-15 induction in macrophages appears to be an exciting prospect as an emerging approach to regeneration immunotherapy,” concluded the researchers.
Future work
According to Andreas Patsalos, M.S., Ph.D., a senior postdoctoral research fellow in the Nagy laboratory and the study’s lead author, macrophages are positioned at the crossroads leading to acute inflammation, tissue repair and regeneration.
“These cells first assume special characteristics to carry out inflammatory functions, then they engage in tissue reparative roles,” Patsalos explains.
He adds that the knowledge gained from their study is expected to offer the researchers a better perspective on just how macrophages and the growth factors they secrete work to heal chronic muscle damage.
These findings, and their future work based on these findings, may provide better treatment for Duchenne Muscular Dystrophy (DMD) and Becker Muscular Dystrophy (BMD). DMD and BMD are rare and often deadly genetic diseases caused by a genetic mutation that prevents the body from producing dystrophin, a protein that muscles require for proper functioning. Without dystrophin, muscle cells become damaged and weaken over time. There is no cure.
“Current treatment for DMD and BMD needs improvement, and there is a strong motivation for researchers at Johns Hopkins All Children’s to discover novel pathways to help extend or even save the lives of children with muscular dystrophies,” says Patsalos. “Our goal is to find ways to support the repair of acute and chronic muscle damage. That means identifying the right type of macrophages and the ‘switches’ on them, called ‘transcription factors,’ that transition them toward healing. We want to be able to modify and enhance their activity. That is the goal and the next step. We are already working on it.”