Meet Our Investigators
The John Fetting Fund is an endowment that supports basic and translational breast cancer prevention research performed by or under the direct supervision of the faculty of the Johns Hopkins Breast Cancer Program. The priority of the Fund is genomic and epigenomic research, which distinguishes women at-risk for breast cancer and its consequences from women not at risk. By refining our understanding of who is at risk, we can focus expensive and involved health care on those at risk and reassure those who are not. The Fund supports preliminary studies which position investigators to secure larger grants. The Fund will also provide stopgap or interim funding for promising work that has run out of funding while the investigator seeks more substantial funding.
Learn More About Our Investigators
Understanding the Biological Consequences of Dense Breast Tissue In Order to Prevent Breast Cancer - Dr Daniele Gilkes
Advancing Early Detection of Breast Cancer Using Liquid Biopsies - Dr Emily Ambinder and Dr Jessica Tao
Oral microbiota, A Modifiable Breast Cancer Risk Factor, In Women Harboring Genetic Mutations - Dr Dipali Sharma
Repurposing the Anthelminthic, Mebendazole For Prevention of Breast Cancer - Dr Sara Sukumar
Incorporating Novel Biomarkers Into Tools For Risk Assessment - Dr Kala Visvanathan
- Understanding the chemical alterations to DNA, a process known as DNA methylation. These alterations can change the function of specific genes and allow cancer to develop, making them a promising tool for early breast cancer detection.
- Testing for cell-free or circulating tumor DNA (ctDNA) in the blood has the potential to identify individuals with breast cancer before it can be seen in imaging or other tests, guide prognosis and treatment for patients with newly diagnosed breast cancer, and identify women at increased risk of developing breast cancer.
- Using a compound called Honokiol, which comes from plants, in combination with a drug called Chloroquine may help prevent breast cancer.
- Studying biomarkers that can help identify women who may not be benefiting from the chemo-preventative properties of the breast cancer drug tamoxifen.
- Uncovering the changes in the gut and/or breast bacteria (microbiome) which may modulate breast cancer initiation and response to therapy.
- Continuing laboratory studies of an intraductal injection called IT that destroys all cancer cells in laboratory models and remains protective for more than five months after the treatment.
- Understanding how alcohol increases a woman’s risk of developing breast cancer and which women may be more susceptible.
Support Our Team
More About Our Fetting Fund Investigators
-

Dr. Visvanathan is the first Fetting Scholar and an expert in breast cancer prevention. Her team has conducted several studies.
Mapping Methylation Changes in the Breast
Chemical alterations to DNA, a process known as DNA methylation, can alter the function of specific genes, allowing cancer to develop, and may also be a promising tool for early detection of cancer. The research explores whether fine needle sampling, which uses a tiny needle to extract a sampling of cells from the breast, could be used to detect the presence of DNA methylation or precancer cells in breast tissue and predict risk of breast cancer development.
In 20 women diagnosed with breast cancer, the team measured DNA methylation in the tumor and noncancerous cells from seven different areas of the breast and opposite breast. The study involved 16 Johns Hopkins clinicians and scientists. High levels of DNA methylation in cancer cells. To our surprise, we did not detect high levels of methylation in breast tissue next to the tumor or in the other quadrants, except in women with a strong family history of breast cancer. However, the fine needle sampling method did not detect small early methylated breast lesions found on pathology review. These lesions were also undetected on imaging.
Our results suggest that fine needle samples are not an effective approach to detect and assess DNA methylation unless there is already a known tumor. However, the innovative design of the study enabled us to quickly determine the potential utility of this approach. In other ongoing studies by our research team, methylation levels appear to be a promising marker to distinguish benign tumors from malignant tumors and to predict disease progression in women with metastatic disease. We are in process of resubmitting a manuscript for peer review.
The Impact of Risk-Stratification on Breast Screening Adherence
Description automatically generatedSince 2007, U.S. national guidelines recommend that cancer-free women found to have a with 20% or higher lifetime breast cancer risk receive yearly screening with mammogram and magnetic resonance imaging (MRI). The team examined screening utilization and adherence to screening recommendations over time in 374 women, 30 years or older, who had no history of breast and ovarian cancer and had, who visited the Johns Hopkins Cancer Genetics Clinic for risk assessment between 2004-2013. Women with lifetime risk of 20% or higher were considered high-risk, and those with lifetime risk under 20% were considered low risk. Lifetime risk and adherence to breast cancer screening guidelines were evaluated at four and eight years.
The team reported that less than 35% of high-risk women utilized breast MRI over eight years. High-risk women were 85% less adherent to guidelines compared to low risk women at four years, mainly because of due to the poor adherence to recommended breast MRI. More than 78% of women reported appropriate screening for other cancers. Predictors of non-adherence included younger age, fewer additional health problems, and no recent clinical breast exam. Based on predicted probabilities of adherence, only 450 of 1,000 high-risk, educated, mothers, 50 years or older with additional health issues, would adhere at four years.
Our results suggest that most high-risk women were not adherent to increased surveillance. Studies focused on removing barriers to low MRI adherence are needed for these women to benefit. Although,
75% to 85% of high-risk women did not adhere to MRI screening guidelines at four and eight years, most of these women, received mammography and screening tests for other cancers during the same time. -

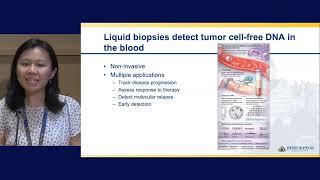
Pilot Study to Evaluate the Role of Circulating Tumor DNA in Breast Cancer Detection
Currently, the guidelines for breast cancer screening are controversial because there is inconsistency in recommendations among the different professional societies. Adding to the confusion is differing opinions on the optimal screening for women at increased risk for breast cancer, such as those with a predisposing genetic mutation (i.e. BRCA 1 or 2 mutation) or dense breast tissue. In this era of Precision Medicine, it is time to move past the “one size fits all” approach to breast cancer screening. Testing for cell-free, or circulating tumor DNA (ctDNA) in the blood, has the potential to identify individuals with breast cancer before it can be seen in imaging or other tests, guide prognosis and treatment for patients newly diagnosed breast cancer, and identify women at increased risk of developing breast cancer.
The team initiated a pilot study of individuals with a breast abnormality to determine the potential role of ctDNA breast cancer detection and prevention. Women receiving a breast biopsy will provide a blood sample before biopsy to evaluate the accuracy of ctDNA for breast cancer detection. Findings from ctDNA tests will be compared to and evaluated with imaging and pathology. This is a first step in a multiyear project where Johns Hopkins will become a leader in the use of ctDNA in breast cancer detection and prevention -

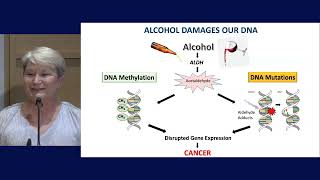
Identification of Alcohol-Induced Alterations and Increased Risk of Breast Cancer
A study from the World Health Organization reported that more than 100,000 cases of breast cancer diagnosed in 2020 were attributed to alcohol consumption. This means that about 4% of breast cancers worldwide may be preventable. However, few people--about one-third of people according to experts--recognize the association between alcohol and breast cancer. Awareness is also low among health care providers, researchers report, signaling a need to inform women of the dangers associated with alcohol consumption and to determine which women are most at risk and should avoid alcohol.Research to identify how alcohol alters the DNA of normal breast cells to induce unwanted, malignant behavior will inform this risk assessment. In collaboration with Dr. Dipali Sharma, Dr. Zahnow’s team demonstrated that normal breast cells derived from a healthy woman can be dramatically altered by exposure to alcohol. Normal breast cells treated with alcohol are able to transform and spread, behaviors that are usually associated with cancer cells. The team will next study the behavior of alcohol-treated cells in other laboratory models.
The research team also demonstrated that alcohol exposure can alter the DNA chromatin architecture. Chromatin, is a complex combination of DNA and proteins, mainly histones, and it’s job is to compress DNA so it fits inside cells. How chromatin is packaged inside cells--loose or tight-- plays a major role in gene regulation and cellular behavior. The researchers observed that alcohol exposure leads to more open chromatin near the important regulatory regions of genes. They have also observed significant changes in DNA methylation (a chemical alteration to DNA) after alcohol exposure. DNA methylation can regulate gene expression and often tends to silence genes that are important for suppressing tumor growth.
These preliminary data suggest that exposure to alcohol leads to changes in the chromatin and methylation of DNA that are also observed in some women with non invasive breast cancer. Using our bioinformatics data and various data sets from the US, UK and the Netherlands, they plan to test whether alcohol consumption contributes to epigenome characteristics which can distinguish indolent from aggressive DCIS. Our promising work is taking us one step closer to understanding how alcohol increases a woman’s risk of developing breast cancer and which women may be more susceptible.
-

Honokiol as a Chemopreventive for Breast Cancer
The laboratory’s recent studies showed that Honokiol treatment induces a mechanism known as cytoprotective autophagy, which may protect cancer cells to some extent. To overcome this, they propose using Honokiol in combination with Chloroquine to improve its anticancer potential. The research team continues to work to develop this compound, and are planning to submit a proposal to the NCI-PREVENT program to support further development.
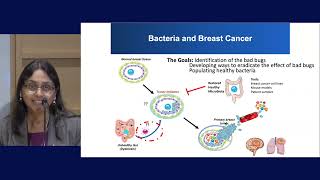
Bacteria In Gut and Breast
Breast cancer can develop in women with no apparent risk factors, and women with similar clinical profiles respond differently to prevention and treatment strategies. The team is working to uncover the changes in gut and/or breast bacteria (microbiome) which may modulate breast cancer initiation and response to therapy. The team is collecting stool samples from breast cancer patients to analyze their gut bacteria. These studies will uncover important bugs and establish us in the microbiome-breast cancer field.
Obesity and Breast cancer
Tamoxifen has been used as a chemoprevention agent for breast cancer. Our recent study explains why some obese women with ER-positive breast cancer may not get the full benefit of tamoxifen. Ongoing research focuses on biomarkers that can help identify obese women who may not be benefiting from Tamoxifen. -

First-Of-Its-Kind Prevention Strategy
Description automatically generatedA promising new prevention strategy uses a new agent--recombinant immunotoxin (IT), HB21-(Fv)PE40, a fusion protein of the transferrin receptor linked to a fragment of a bacterial toxin. Breast cancer cells-- even in their earliest stages--express the transferrin receptor abundantly, and as a result internalize the receptor and the toxin with it, which results in immediate death of the tumor cells with no effect on normal cells. In two mouse models of DCIS our research showed that intraductal injection of the IT destroys all cancer cells and protects them for more than five months after the treatment. This level of clearance of tumor cells and the sustained protective effect of the IT has never been observed before with any other preventive agent. Since the toxin is potent, side effects could be substantial when injected intravenous into the bloodstream. Using the intraductal route we tested, tumors died, and not even trace amounts of the IT were detected in the circulation of the mice. These findings are currently being prepared for publication
Fetting Fund Investigator Panels
Fetting Fund Founders Dr. John Fetting and Leslie Ries
Fetting Fund Investigator Dr. Cindy Zahnow
Fetting Fund Investigator Dr. Dipali Sharma
Fetting Fund Investigator Dr. Jessica Tao
Fetting Fund Investigator Dr. Gilkes
Fetting Fund Investigator Dr. Vered Stearns
Fetting Fund Investigator Dr. Vered Stearns 2nd Session
Fetting Fund Panel Discussion