An Immunotherapy Pioneer
Research nurse Alice Pons recalls the first clinical trials of immunotherapy drugs
 PONS
PONSAlice Pons is a pioneer in cancer immunotherapy. In 2007, she administered some of the first treatments in clinical trials of anti-PD-1 and anti-PD-L1 immunotherapies.
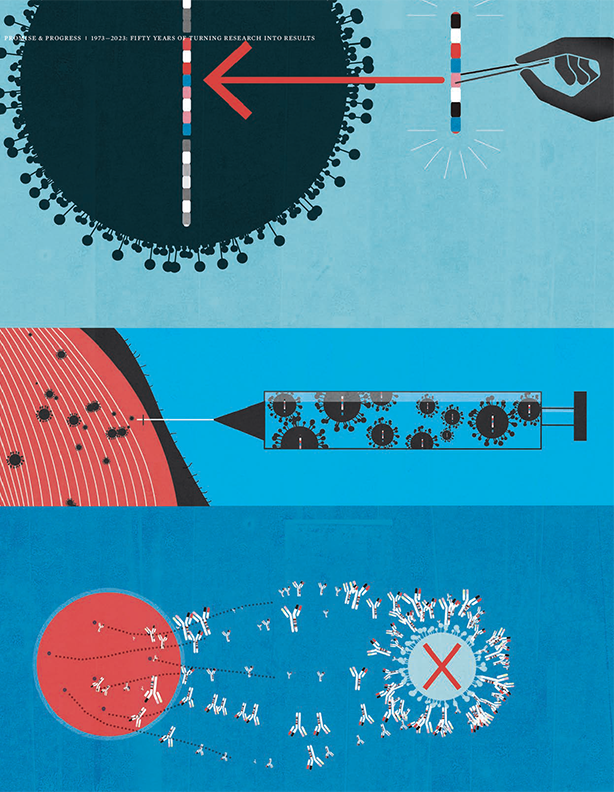
These new cancer therapies were developed based on research at the Johns Hopkins Kimmel Cancer Center that showed cancer cells co-opted natural on/off switches of the immune system, called immune checkpoints, to shut down the immune response to cancer. The new drugs, researchers believed, had the power to re-ignite the immune response.
Ultimately, they transformed the cancer treatment paradigm, mainstreaming immunotherapy as a cancer medicine. However, in 2007, they were trailblazers, as Phase I clinical trials began to evaluate the safety and determine the dosing for these promising new drugs that were about to be given for the first time to patients.
New therapies showed that cancer cells co-opted natural on/off switches of the immune system, called immune checkpoints, to shut down the immune response to cancer. The new drugs, researchers believed, had the power to re-ignite the immune response.
Alice recalls administering the first doses of the drugs in patients with advanced cancers who were out of options. Many of them were near death, she recalls.
“Desperation,” is the word that best describes the mood, she says. “These patients had tried a lot of therapies that did not work against their cancers. This was their last chance.”
They wanted the drug to be the thing that finally worked for them, but if it didn’t, maybe what the nurses, doctors and researchers learned from them would help others in the future. Patients wanted to find a greater meaning in their battle against cancer, for them and for their families, says Alice.
The anti-PD-1 and anti-PD-L1 immunotherapies were different than the chemotherapies that had led to the dreadfully common characteristics that grew to define cancer, and the fear associated with the disease. The treatments, while lifesaving for many, often came with debilitating side effects. Essentially, they worked by poisoning rapidly reproducing cells, which meant they killed cancer cells, hair cells, gut cells and more, causing patients to lose their hair and suffer from nausea and vomiting. It is not an overstatement to say many patients feared treatment as much as the disease.
The patients Alice was treating in these immunotherapy clinical trials had already received many rounds of chemotherapy. “They were worn out,” she says. “When we told them these drugs did not usually cause hair loss or nausea and vomiting, they were ready to give it a try.”
From the very first infusion, it was clear this treatment was different, Alice says. It truly was a new frontier. They didn’t know what to expect, so they were hyper vigilant.
“We took vital signs every 15 minutes,” says Alice. “Almost immediately, we could see this was not like chemo. When you give chemo, patients get sick. With this, they did not.”
It took a year before they began to see responders. The immunotherapy drugs worked best against melanoma, a type of lung cancer called non-small cell lung cancer, and kidney cancer.
Alice says they were elated when some patients began to respond to the immunotherapy. “I would be sitting with the different doctors when they got the results that it was working. They couldn’t wait to tell the patient. Of course, the patients were very happy,” she says.
Then, she says, trepidation set in, and we began to worry about how long it would last. Would we get to the next scan and find the cancer had come back?
Alice worked so closely with patients and families, it was impossible not to become emotionally invested. She was beyond excited about the responses but admits she remained skeptical.
For Alice, it wasn’t about the science, the proof was in the patient standing in front of her.
“We didn’t know why it worked in melanoma and a few kidney cancer and lung cancer patients but not in other cancers. I was stoked for the melanoma patients because they had been striking out for so long,” says Alice. “I was so deep in the trenches with the patients that sometimes the broader picture eluded me. The doctors were confident. They would tell me, it was going to work, but I found it hard to believe until it a happened a couple of times over a couple of scans.”
As more responses to the treatment became long lasting, extending years, her relationships with the patients and families did as well. This was unusual because most patients with advanced cancers died within months. She was invested.
“Every time a patient came back for a scan, I would get a pit in my stomach. ‘What if it comes back?’ I’d think to myself, and I knew the patient had the same worry.”
Her thoughts immediately turn to a patient whose cancer came back after two years. “It was heartbreaking. I had let my guard down and believed the immunotherapy would make it a lasting remission,” she says.
Fortunately, for many patients, there have been long remissions. In fact, some have lasted 15 years and are considered cured, says Drew Pardoll, M.D., Ph.D., Director of the Bloomberg~Kimmel Institute for Cancer Immunotherapy.
Still, one of the key areas of research remains focused on figuring out who will respond and then among responders what makes the treatment stop working.
“There is much left to learn,” says Dr. Pardoll. “We’re getting better at predicting who will respond in the first place, but we still don’t know why some people who respond relapse years later.”
Another area of major focus, and again one where the Bloomberg~Kimmel Institute for Cancer Immunotherapy led the way, was in detecting and managing side effects. The immunotherapy drugs worked by unleashing the immense power of the immune system, and sometimes the immune system attacked healthy tissue and organs in addition to cancer cells.
Just as this type of immunotherapy was new, so were its side effects. They were not as obvious as chemotherapy side effects.
“They crept up and were harder to detect,” says Alice. “They were outside of my realm of experience having done chemo for so long.”
“We can’t minimize the autoimmune side effects because there were people who sacrificed their lives on the way because they were already suffering from toxicities cause by chemotherapy, and now on top of that, they autoimmune side effects,” says Alice. “We got hit pretty quickly with what worked and didn’t work and with warning signs about something coming and heading it off at the path. It was hard in the beginning, but we figured out fast how to recognize warning signs and how to manage them.”
When the Bloomberg~Kimmel Institute-developed therapies were proven in clinical trials, received FDA approval, and now were being administered by community practices, the importance of recognizing and managing side effects became even more evident.
“We had patients coming to us because their immunotherapy was poorly managed in the community.”
Managing side effects was a science of its own that many beyond the walls of the Bloomberg~Kimmel Institute did not appreciate. Our experts engaged a broad range of specialists—endocrinology, rheumatology, dermatology, and more—to develop the standard of care and ensure doctors and patients recognized the warning signs, which to an untrained eye could be easy to miss.
In general, serious side effects occur in about 20% of patients, but at the Bloomberg~Kimmel Institute, it’s much lower at 5% to 10%. Alice says it is the expertise here that makes the difference.
Alice credits the doctors and scientists who developed the treatment protocols for making Johns Hopkins a standout.
“Had I not been on this team, with Suzanne Topalian at the helm, it would have been a lot different. She kept our noses to the grindstone and instilled in all of us the importance of focus and attention to detail. I am very thankful for that. This strong leadership and commitment to patient care is what makes the Kimmel Cancer Center and the Bloomberg~Kimmel Institute special.”
“On a clinical trial, we’re giving them concierge nursing. I’m responsible for my patients. I want to know; I have to know. I go through the side effects from head to toe with each patient. We’re calling, emailing. That accessibility translates into patients being treated for their side effects more quickly. Our goal is to keep everyone safe,” she says.
Fast forward to 2023, and now combination therapies of different immunotherapies, and even chemotherapy, given together are improving response rates. Research at the Bloomberg~Kimmel Institute has led to multiple FDA approvals and immunotherapy as a first line treatment for many types of cancer.
Alice becomes emotional when she thinks of the journey from the very first patient she treated to the continuing progress still being made today.
“It was exhilarating. I imagine it is how skydiving feels. In a way, that’s what we were doing,” she says referring to all of the unknowns of the first clinical trials. “It achieved one of my objectives in life, to contribute to mankind. This allowed me to do it, and I am forever thankful.”