Tumor Clues
Tracking Molecular Cancer Footprints in Blood
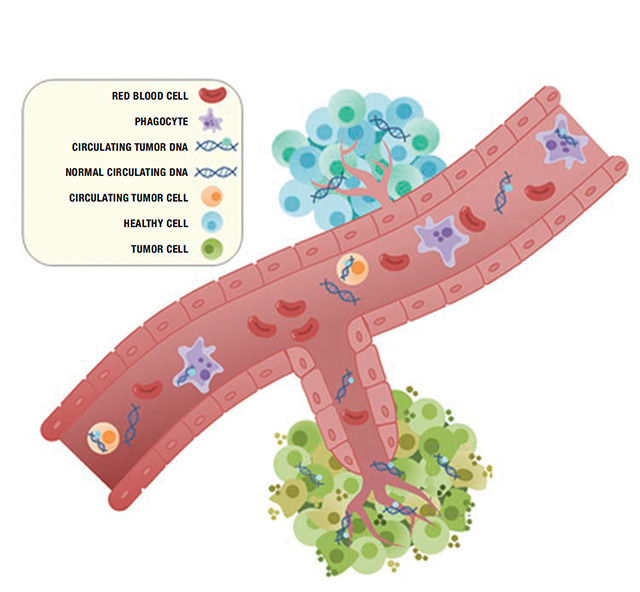

As tumor cells die, they leave fragments of their genetic material (also known as DNA) circulating in the bloodstream. This molecular footprint, known as circulating tumor DNA, can be detected in a simple blood test by ultra-sensitive technologies such as next-generation sequencing, known as liquid biopsies. Johns Hopkins Kimmel Cancer Center researchers were among the first to point this out and to study how it could be used to detect cancer earlier and to guide cancer treatment.
Valsamo Anagnostou, M.D., Ph.D., director of the thoracic oncology biorepository at Johns Hopkins, believes this technology could help patients with esophageal cancer and is collaborating with oncology experts Joy Feliciano, M.D., medical director of the thoracic oncology program at Johns Hopkins Bayview Medical Center, and Vincent Lam, M.D., director of the esophageal cancer research program.
Looking for circulating tumor DNA in the bloodstream after surgery with the liquid biopsy blood test can help inform doctors if microscopic cancer cells invisible to the surgeon’s eye remain. These cells (micrometastases) are critical because they can cause the cancer to come back and spread to other parts of the body.
“It is a way of tracking in real-time the status and extent of the cancer,” Anagnostou explains.
If the doctors find this molecular footprint — tumor DNA circulating in the bloodstream — or what Anagnostou calls molecular persistence, they know cancer remains. They may opt for more aggressive therapies or different drugs to kill lingering cancer cells. Patients who do not have any circulating tumor DNA in the blood after surgery — Anagnostou calls this molecular clearance — are at low risk for cancer recurrence and could be spared additional therapies and the side effects that frequently accompany them.
“Maybe patients who show sustained circulating tumor DNA clearance are less likely to require chemotherapy, radiation therapy or immunotherapy,” says Anagnostou. Another approach, she says, is to use circulating tumor DNA to help determine if patients have received enough treatment, reserving more aggressive treatments for patients with detectable circulating tumor DNA and adjusting courses and types of therapy until tumor DNA in the blood is no longer detectable.
Anagnostou hopes that monitoring circulating tumor DNA can help guide how to best alternate and sequence therapies to improve clinical outcomes.
Her research is providing new clinical evidence. In early findings from 16 of 32 patients with esophageal cancer who are participating in a clinical trial in which many blood samples were collected over time, Anagnostou says they were able to demonstrate that patients who had a molecular response (no circulating tumor DNA after immunotherapy, chemoradiation and surgery) had better clinical outcomes. On the other hand, patients who had molecular persistence (circulating tumor DNA after immunotherapy, chemoradiation and surgery) were more likely to have a cancer recurrence. These findings were discussed at the American Association for Cancer Research annual meeting in 2021 and reported in the Journal of Clinical Oncology on Feb. 1, 2022.
“These findings are very relevant for esophageal cancer because these approaches provide a noninvasive way to detect cancer recurrence and monitor therapeutic response,” says Anagnostou.
Several challenges remain, she says, before this approach can become a standard practice in cancer care. Next-generation sequencing assays may be limited in detecting very low levels of circulating tumor DNA in patients with early stage esophageal cancer. Furthermore, not all DNA, and changes in its sequence (mutations), in the bloodstream comes from the tumor, she says. Emerging technologies are helping to sort out these challenges.
Since circulating tumor DNA is tracked through a simple blood draw, use of liquid biopsies may expand eligibility and participation in clinical trials for patients.
“Most clinical trials are conducted at academic centers like ours, but blood samples can be easily and safely collected locally and shipped to us, making clinical trials and cancer care more inclusive,” she says. “This is the future.”
