Esophageal Cancer Screening
DNA Biomarker Panel May Become Screening Test for Esophageal Cancer

Stephen Meltzer, M.D.
As director of the Gastrointestinal Early Detection Biomarkers Laboratory, Stephen Meltzer, M.D., is collecting clues that already exist in the esophagus to detect esophageal cancer.
“With esophageal cancer, diagnosis is a matter of life and death,” says Dr. Meltzer, who has dedicated decades of his career to the detection and prevention of this disease. “Currently, there is no inexpensive, minimally invasive screening tool that can be used regularly to diagnose those at risk for esophageal squamous cell cancer.” This includes people with exposure to tobacco, wood smoke, (common in some low-income countries), alcohol, environmental carcinogens, or other esophageal squamous cell cancer risk factors, he says.
Worldwide, 604,000 people are predicted to develop esophageal cancer this year. Esophageal squamous cell cancer occurs in the cells that line the esophagus and is by far the most common type of esophageal cancer.
The challenge, Dr. Meltzer says, is that there are currently no screening tests to find this common type of esophageal cancer in people without symptoms, in contrast to other cancer types. For example, we have screening mammography for breast cancer screening, colonoscopy for colorectal cancer screening, serum PSA for prostate cancer screening, and Pap smears for cervical cancer screening. Moreover, if someone has symptoms suggesting esophageal squamous cell cancer, they must undergo endoscopy and biopsy, which are expensive, risky, and often unavailable in developing countries, where the risk for the disease is highest, he explains.
Most importantly, this lack of screening means that most esophageal squamous cell cancers are detected at very late stages, with the resulting five-year survival rate less than 20%. Since esophageal cancer incidence is now rapidly rising, Dr. Meltzer is responding to an urgent worldwide need with a safe, inexpensive, easy-to-administer screening test.
Building on a nearly 30-year-old discovery by Kimmel Cancer Center epigenetics experts, Dr. Meltzer developed a noninvasive DNA-based test that detects cancer in the lining of the esophagus. “Epigenetics” refers to chemical changes in DNA that can serve as telltale clues, or biomarkers, that occur in cells lining the surface of the esophagus and signaling the presence of cancer.
To capture esophageal cells, Dr. Meltzer uses a device consisting of a small gelatin capsule containing a soft sponge, about as long as the diameter of a dime, attached to a flexible string. The end of the string remains outside the mouth. A few minutes after being swallowed, it dissolves in the stomach; It is then pulled out via the string, collecting esophageal lining cells as it moves upward and out of the mouth.
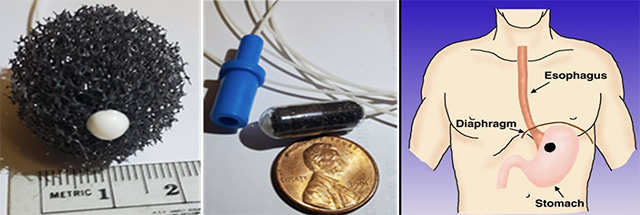
A statistical algorithm specific to esophageal squamous cell cancer is then applied to measure and analyze the collected cells for levels of DNA methylation of specific genes, which occur predominantly in cancer cells.
In a recent study, Dr. Meltzer’s research team, which included Drs. Joy Feliciano, Vincent Lam, Richard Battafarano, Kristen Marrone, several Johns Hopkins University gastroenterologists, JHU engineers, epidemiologists, statisticians, and other key national and international collaborators, administered the test to 94 people. Participants included patients at Johns Hopkins Hospital, but prominently featured many patients in Uganda, which has high rates of esophageal squamous cell cancer. In this study, the test successfully classified about 90% of patients as having esophageal squamous cell cancer or not.
“These findings have global implications, with a large impact on patients in lower-income countries who have limited access to health care resources,” says Dr. Meltzer. “We may be able to save thousands of lives if we can detect this disease early enough to intervene therapeutically.”
Dr. Meltzer hopes to soon bring tests to market for detecting all types of esophageal cancer and their precancerous precursor conditions, through a company he co-founded called Capsulomics, Inc.
Dr. Meltzer says these preliminary results suggest that larger screening trials should be conducted in high-risk populations around the world. He is hopeful the test may also help guide surveillance for recurrence after primary cancer treatment
