Timothy F. Witham, MD

- Director, the Johns Hopkins Neurosurgery Spinal Fusion Laboratory
- Professor of Neurological Surgery
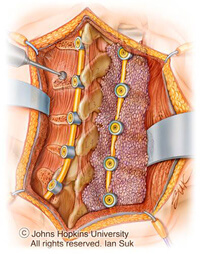
Fusion surgery can treat several problems with the spine, including those arising from trauma, inborn deformity, degenerative disease, infection and tumors.
But 5% to 35% of these procedures fail, even when using the “gold standard” treatment of grafting bone from the patient’s own iliac crest (the top part of the hipbone).
Fusion failure, otherwise known as pseudoarthrosis, is a major cause of failed back surgery syndrome and results in significant pain and disability, increasing the need for more procedures and driving up health care costs.
The ultimate goal of the Johns Hopkins Spinal Fusion Laboratory is to eliminate pseudoarthrosis by using animal models to study various strategies to improve spinal fusion outcomes, including:

Spinal fusion in our animal models is assessed using a combination of complementary techniques incorporating manual palpitation, X-ray and computed tomography (CT) imaging, histology and biomechanical testing.
Our laboratory is also interested in problems regarding overall spine health. In particular, we investigate therapies to improve vertebral bone quality in patients with conditions such as osteoporosis that may require future spinal fusion procedures.
We are developing an animal model to explore the effects of localized radiation therapy on spinal structure and stability, and testing potential therapies for the prevention of vertebral compression fractures in spinal oncology patients.
Dr. Timothy Witham and his team of researchers are investigating spinal fusion surgery outcomes. Their goal is to eliminate fusion failure, which occurs when the bone and the spine do not grow properly and fuse together.


Dr. Sarkar joined Witham Lab in the Department of Neurosurgery as a postdoctoral fellow aiming to advance spinal fusion outcome in aging patients using patient-specific immunomodulatory biomaterials. Originally from India, she completed he undergraduate degree in pharmacy before moving to South Korea to earn a Master's in Materials Science and Engineering. She received her Ph.D. from Washington State University, where she developed 3D-printed tissue engineering scaffolds with controlled release of plant-derived biomolecules for osteogenesis.
As a postdoctoral fellow at Johns Hopkins Biomedical Engineering Department, Dr. Sarkar used her skillset in medicine, drug delivery, biomaterials and 3D printing to develop custom bone grafts capable of controlled, sustained oxygen release upon implantation. In her current role with the Department of Neurosurgery, she is engineering immunomodulatory spinal fusion grafts and monitoring immune-bone cell crosstalk.
Beyond her research, Dr. Sarkar is passionate about traveling, hiking and all things outdoors. At home, she adores hanging out with her little sidekick, experimenting with new recipes, and sometimes diving into true crime documentaries.

Dr. Shafi is a Doctor of Medicine with roots in Pakistan and Canada. She is interested in neurosurgery, specifically spine fusion and outcomes following spine fusion surgery. After earning her medical degree, Dr. Shafi began researching with the Computational Neurosciences Outcome Centre at Brigham and Women’s Hospital. She later joined the Mayo Clinic Rochester as a postdoctoral fellow. Her time working with these institutions provided her with experience crucial to the investigations conducted at the Spine Fusion Lab at Johns Hopkins.
Dr. Shafi's skills include performing animal surgeries (specifically spine fusions), synthesizing biomaterials, working with cells and writing research papers of clinical and basic science nature. She is excited to use her skillset toward the development of novel therapeutics to advance outcomes in patients undergoing spine fusion.
In addition to her passion for neurosurgery, research and medicine, Dr. Shafi loves to play with makeup, cook, read, travel, work with the underserved of her community, and spend time with family and friends.
Spinal fusion failure, or pseudoarthrosis, is a significant cause of failed back surgery syndrome (FBSS). FBSS occurs in up to 35% of surgeries and often results in chronic pain and disability, which accounts for the interest in developing pharmacologic strategies to improve fusion rates in initial procedures.
The only therapeutic agent currently approved by the Food and Drug Administration for spinal fusion surgery is recombinant human bone morphogenetic protein-2 (rhBMP-2), a growth factor in the bone morphogenetic protein (BMP) family that has been shown to improve fusion rates in clinical studies. Although generally successful in augmenting fusion, rhBMP-2 treatment has been associated with complications and adverse effects.
The Johns Hopkins Spinal Fusion Laboratory is interested in developing improved therapeutic delivery techniques for spinal fusion procedures, including strategies involving the combined delivery of BMP family proteins and other biological agents.
We are studying the effects of the osteoporosis drug teriparatide (a parathyroid hormone analog) on spinal fusion in an animal model and in combination with BMP-2 delivery. Future work will explore using other bioactive compounds and alternative delivery strategies for better control of delivery kinetics.
Traditionally, radiation therapy has been used to treat metastatic lesions in the spine. The advent of stereotactic radiosurgery has enabled clinicians to deliver even higher doses of focused radiation, allowing for noninvasive treatment of spinal tumors previously considered “radiation resistant.”
Stereotactic radiosurgery for these lesions is extremely promising, yet recent clinical studies have shown that the procedure can increase the risk of compression fractures of the vertebrae, especially in patients with osteoporosis. This radiation-related deterioration of bone quality is a critical consideration for spinal oncology patients who require fusion procedures.
We are developing an animal model to study the long-term effects of high-dose, focused radiation on spinal structure and biomechanical integrity. Further, we seek to determine whether fractionated radiation delivery, as opposed to single higher radiation doses, can prevent post-radiation spinal compression fractures.
More important, we will also explore the use of various therapies to mitigate post-radiation fracture risk, including teriparatide. These therapies are used to increase bone density in osteoporotic patients.
In spinal fusion surgery, the current “gold standard” treatment involves autografting bone from the patient’s iliac crest. This technique is limited by bone supply, and is associated with donor site morbidity and a failure rate of 5% to 35%. There is a critical need for bone graft substitutes that can also reduce the rate of pseudoarthrosis.
Tissue engineering is a promising new strategy. By combining osteoconductive carriers, which support bone growth, with growth factors that stimulate bone formation or bone-growing stem cells, tissue engineering approaches aim to replicate — or even surpass — the properties of native bone.
Our laboratory is collaborating with Warren Grayson, Ph.D., in the Department of Biomedical Engineering at The Johns Hopkins University, to develop a bioactive “scaffold” system to enhance rates of solid spinal fusion in an animal model. We are also working with the Laboratory for Craniofacial and Orthopaedic Tissue Engineering to study the use of various stem and progenitor cell types in spinal fusion surgery. By seeding our scaffold system with optimal bone-growing stem cells, we aim to achieve solid spinal fusion rates better than those attained with conventional substitutes or even the patient’s own bone.