Laboratory Studies Identify A Potential Way to Treat Human Cancers With ARID1A Mutations
08/19/2019
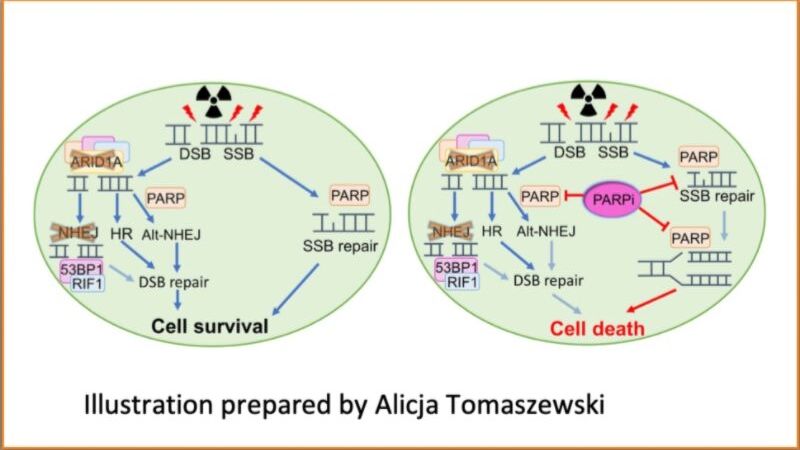
A new study shows that tumor cells depleted of ARID1A — a protein that acts as a cancer suppressor — become highly sensitive to anticancer poly ADP ribose polymerase (PARP) inhibitor drugs after radiation treatment. The research, led by Johns Hopkins Kimmel Cancer Center researchers, could advance efforts to treat many human cancers with loss of ARID1A that are resistant to current standard treatments, the study team suggests.
Previous research shows that ARID1A mutations that prevent cellular production of ARID1A are found in about 50% of ovarian clear cell carcinomas, 35% of uterine endometrioid endometrial adenocarcinomas and 30% of ovarian endometrioid carcinomas. ARID1A mutations are also frequently found in liver, stomach, bladder and pancreas cancers. PARP inhibitor drugs block the enzyme, PARP, that cells use to signal and recruit machinery to repair damage to their DNA, causing tumor cells to die. Cancers with mutations causing defective DNA repair systems tend to be more dependent on PARP than cancers with intact repair systems.
A report on the research was published online June 13 in Clinical Cancer Research.
“ARID1A mutations are highly prevalent in theses human cancers. The goal of the study was to understand what ARID1A normally does in cells and which cellular functions are affected if it is lost due to inactivating mutations,” says le-Ming Shih, M.D., Ph.D., Richard W. TeLinde Distinguished Professor in the Department of Gynecology and Obstetrics at the Johns Hopkins University School of Medicine, and co-director of the Women’s Malignancies Disease Program at the Johns Hopkins Kimmel Cancer Center. “This allows us to develop effective treatments to eradicate cancer cells with ARID1A mutations or functional inactivation.”
Radiation treatment kills tumor cells by damaging the DNA irreparably. If ARID1A-deficient tumors are shown to be responsive to irradiation plus PARP inhibitors in future clinical trials, it could provide a new opportunity to treat several cancer types that do not have many effective therapeutic interventions, such as ovarian clear cell carcinoma, advanced endometrial or stomach cancers, he says.
In their experiments with mouse and human endometrial cancer or normal cells, the researchers deleted ARID1A from cells to determine if ARID1A-depleted cells were sensitive to fractional radiation. Radiation induces DNA damage and is commonly used to treat liver, gastric, bladder and gynecologic cancers. The researchers found that without ARID1A, some of the cells could not recover from radiation damage and died, says Shih.
Those findings prompted the researchers to hypothesize that cells without ARID1A cannot efficiently repair DNA. However, since treatment with radiation alone does not lead to a complete response, the researchers subsequently tested a collection of drugs targeting key enzymes in the DNA repair pathways to find one that might work hand in hand with radiation to make the tumor cells even more vulnerable. Their effort turned up one class of drugs: PARP inhibitors.
The researchers then set up an experiment by establishing tumors composed of human cancer cells in mice and treating the mice with radiation alone, PARP inhibitor alone, a combination of both or no treatment. They found that the ARID1A-deficient tumors shrank significantly when the tumor-bearing mice were treated with combined irradiation and PARP inhibitor but not when treated with either irradiation alone or PARP inhibitor alone. This anti-tumor effect lasted for a prolonged time after treatment. Such a phenomenon was not evident in ARID1A-proficient tumors or normal tissues.
Although radiation treatment causes DNA breaks, cancer cells with intact DNA repair systems are able to fix some of the damage, allowing cancer cells to continue to divide, the researchers say. Because DNA repair is so important in biology, redundant DNA repair systems exist. Two major mechanisms are nonhomologous end joining (NHEJ) and homologous recombination. In ARID1A-mutated cells, this study found that the NHEJ repair pathway was affected, and these cancer cells relied on homologous recombination to maintain tumor growth. Their findings also explained why ARID1A-deficient tumors only partially respond to radiation therapy. When the investigators added a PARP inhibitor to suppress homologous recombination repair after radiation, DNA repair capacity in irradiated ARID1A mutated cancer cells was thwarted, since both the NHEJ and homologous recombination mechanisms were defective.
“Radiation-induced DNA breaks, which cannot be repaired efficiently in ARID1A deficient tumors, prime these tumor cells for enhanced vulnerability to PARP inhibitors,” Shih explains.
Several PARP inhibitors are clinically available and approved to treat BRCA mutation-related ovarian and breast cancers. PARP inhibitors have been tested against ARID1A-deficient tumors before, says Shih, but with limited success against those tumors. The new study may help explain why, he adds.
Given the clinical availability of PARP inhibitors and established clinical benefit of local irradiation, it is expected that the next step will be to determine this combination’s safety and dose through a phase I clinical trial, according to co-authors Akila Viswanathan, M.D., M.P.H., M.Sc., interim director of the Johns Hopkins Department of Radiation Oncology and Molecular Radiation Sciences, and Stéphanie Gaillard, M.D., Ph.D., director of the Gynecologic Clinical Trial Center at Johns Hopkins.
Other researchers were Youngran Park, M. Herman Chui, Yohan Suryo Rahmanto, Zheng-Cheng Yu, Ayse Ayhan, Sonia Franco, Anthony Leung and the co-corresponding author, Tian-Li Wang, at the Sidney Kimmel Comprehensive Cancer Center, and Raghavendra Shamanna, Marina Bellani, Michael Seidman and Vilhelm Bohr at the National Institute on Aging.
The research was supported by the National Cancer Institute, the Gray Foundation and the Richard W. TeLinde Endowment Fund.
